Test for Nitrate Ions
Ions that end in ate have oxygen in them. On the amount of nitrate in the water supply and the balance of other ions in the water.
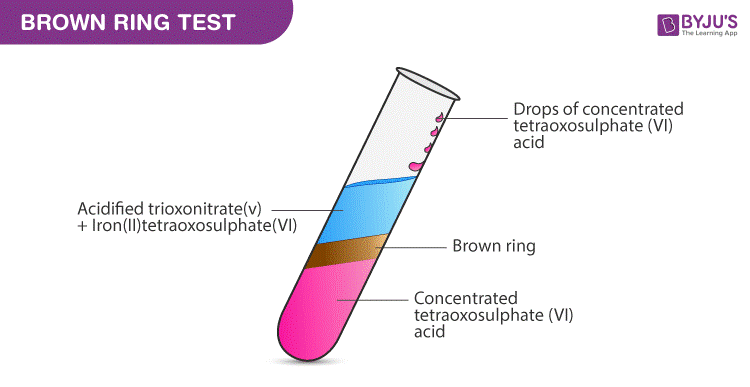
Brown Ring Test Nitrate Test Procedure Reactions On Byju S
In established aquariums the nitrite level should always be 0 ppm mgL.

. Nearly all living organisms from bacteria to humans store iron as microscopic crystals 3 to 8 nm in. Insoluble substance cannot dissociate into ions in water. Silver Nitrate Sticks are a firm wooden stick that contain 75 silver nitrate and 25 potassium nitrate that are used to chemically cauterize the skin.
There are some exceptions. The chemical reaction permanently destroys. Nitrate levels were too high before you changed the water.
If the level is higher than 10 mgL the. Effective anion exchange removal of inorganic nitrate requires softening pretreatment ahead of the anion. Only ionic compounds which are soluble in water forming aqueous solution will dissociate into ions in water.
Silver Nitrate Sticks activate when these two ingredients are mixed with moisture such as a drop of water and cause a chemical reaction when applied to the skin. Scroll down the page for more examples and. So for example if you know chlorate you also know bromate and iodate too BrO3- and IO3-.
The ISE membrane is a solvent-polymer membrane that is a nitrate ion-exchanger in an inert polyvinyl chloride PVC plastic matrix. Investigating transition metal ions. Be sure to test whether the filter is nitrate-free.
Observe and record the colour of any precipitate formed. However arsenate is like phosphate. Similarly the photolysis of DOM in the presence of halide ions can be an alternative and significant pathway to generate reactive oxygen and halogen radicals for inorganic Mn.
The ion exchange process for example is sensitive to waters containing high TDS high sulfate and high hardness levels which can cause hardness precipitation during regeneration. Nitrate compounds for gunpowder were historically produced in the absence of mineral nitrate sources by means of various fermentation processes using urine and dung. Chloride ions will produce a white precipitate bromide a cream precipitate and iodide produces a yellow precipitate.
Lightning strikes in earths nitrogen- and oxygen-rich atmosphere produce a mixture of oxides of nitrogen which form nitrous ions and nitrate ions which are washed from the atmosphere by rain or in occult. If copper iron or other metals are present in concentrations above several mgL the reaction with the cadmium will be slowed down and the reaction time will have to be increased. Add 2 ml of Tollens reagent to both the test tubes.
Elements in the same family make similar ions. It is very important that any unreacted hydroxide ions are removed completely to give a neutral or acidic solution as silver hydroxide will also produce a precipitate. This water should not be consumed until corrective action is taken.
The following diagram shows how to write the ionic equation for the reaction of aqueous sodium carbonate with aqueous barium nitrate. Almost all known forms of life particularly complex life require iron. To tell whether an unknown substance contains ironII nitrate or ironIII nitrate add a few drops of sodium hydroxide solution.
This test has to be done in solution. Nitrate is not like phosphate even though nitrogen and phosphorus are in the same group. Water with Nitrate-Nitrite as N less than 10 mgL is considered safe for human consumption.
Take two clean dry test tubes and add 1 ml of the test sample in one test tube and 1 ml of distilled water in another as blank. As the biological filter becomes established in 4 to 6 weeks the nitrite levels will drop to 0 ppm mgL. Procedure of Tollens test.
The reagents used for this method are often prepackaged for different. In new aquariums the nitrite level can gradually climb to 5 ppm or more. Keep both the test tubes in a water bath for 1 min.
If a sample is turbid it should be filtered through a 045-micron filter. The maximum contaminate level for Nitrate-Nitrite as N in drinking water as determined by the EPA is 10 mgL or parts per million ppm. Test for halide ions Add a few drops of dilute nitric acid then a few drops of silver nitrate solution.
Nitrates are ions NO 3- and fish can gradually adapt to change in the level of ions and. The presence of nitrite in established aquariums indicates. However the sudden drop of Nitrate levels that follows a water change could send your fish into Osmotic Shock.
The reactive halogen radicals were generated from nitrate photolysis in the presence of naturally abundant halide ions which facilitated the oxidation of Mn 2 aq to δ-MnO 2 nanosheets. A high level of Nitrate in the aquarium water can be inherently toxic to fish and may even kill them. If you start from a solid it must first be dissolved in pure water.
The solution is acidified by adding dilute nitric acid. Those are an important subclass of the metalloproteinsExamples include oxyhemoglobin ferredoxin and the cytochromes. Many proteins in living beings contain bound ironIII ions.
Silver nitrate dilute nitric acid The nitric acid reacts with and removes other ions that might also give a confusing precipitate with silver nitrate. NITRITE NITRATE TEST What the Test Results Mean Nitrite. The halide ions can be detected using nitric acid followed by silver nitrate.
The absorbed nitrate ions cause a potential voltage that is proportional to the concentration of nitrate in the sample. Observe the formation of color and note it down. Nitrate ions are selectively absorbed by the ISE membrane.
The nitrate electrode has an internal silversilver chloride. While in La 3-ZS electrolyte both the bivalent Zn 2 ions and trivalent La 3 ions are absorbed on the surface of Zn deposits resulting in fewer net negative charges than that in.

Iit Jee Individual Test Nitrate Ion No3 Offered By Unacademy

In The Ring Test Of No 3 Ion Fe 2 Ion Reduces Nitrate Ion To Nitric Oxide Which Youtube

Question Video Testing For The Presence Of Nitrate Ions Using The Brown Ring Test Nagwa
Comments
Post a Comment